Student name:_________________________________________
CHEM
1079 Analytical Science
Practical:
Voltammetry Simulation
Due date:
The program Polar41c has been
downloaded onto the computers in the
Department's computer lab.
You can download a copy yourself (as a zip file) from http://www.DrHuang.com, unzip and install it on your own
computer. Note that the program is
too large to fit on a floppy disc.
In this exercise you will
perform a number of experiments on a voltammetry simulator. You may not have covered this material
in lectures and should refer to your CHEM 1079 lecture notes and textbook
(Harris, Chapter 18).

DC Voltammetry

If
an electrochemical reaction occurs, electrons are transferred and a current
flows. The current is
measured as a function of the potential applied to the electrode. A voltammogram is a plot of the
current, i, on the vertical axis versus the potential, E, on the horizontal
axis. Cathodic (reduction) currents
are shown in the positive y-direction and negative potentials are shown in the
positive x-direction.
In this series of experiments
we are looking at the two-electron reduction of Cu2+ to Cu. The Eo of the reduction is
+0.34 V relative to the standard hydrogen electrode, or +0.10 V relative to the
saturated calomel electrode, SCE, (which is more commonly used as a reference
in voltammetry).
If we apply a potential
greater (more positive) than 0.10 V vs. SCE, the reaction
Cu2+
+ 2e ® Cu
will not occur. In voltammetry of metal ions, we start
with an applied potential well in excess (more positive) of the Eo
of the metal ion present and scan the potential in the negative direction as a
linear function of time. As the
potential approaches 0.10 V, the reaction above begins to occur and a current
flows. As the potential is brought
closer to 0.1 V, this current increases until, a little way past 0.1 V, it
flattens out. See for yourself:
Load the program from
Windows:
·
Start/ Programs/ Polar/ Polar
·
Maximise
Now run the simulation:
·
Input/ Techniques ®
DC voltammetry
·
Input/ Mechanism ®
A + 2e®B
·
Run/ Simulate
Sketch the polarogram (i versus E):
Note the gradual increase of
current with voltage around the Eo. Current (coulombs per second) is a measure of the rate
of the electrochemical reaction.
The reaction starts slowly at first but is driven faster as the voltage
is increased. Note also the
levelling off of the current to form a plateau.
What causes this
plateau? As Cu2+ ions
near the surface of the electrode react to form Cu (that is deposited on to the
electrode), the region close to the electrode is
depleted of Cu2+
ions. We have
to wait for more Cu2+
ions to diffuse from the bulk
solution (where they are in high concentration) to the electrode surface (where
they are in low concentration) before the reaction can continue. The rate of
reaction, and hence the current, becomes limited by the rate of diffusion of
ions from the bulk of the solution to the electrode surface, and the current
plateaus.
There are a couple of
parameters that can be obtained from the voltammogram that are of interest to
us. The diffusion current , id, is the (diffusion) limited current
at the top of the voltammogram.
The half-wave potential, E1/2, is the potential at id/2.
Find the values of id
and E1/2:
·
Analysis/ Find Halfwave E
You can put gridlines in if
you want:
·
Display/ Option ®
Grid
Show
id and E1/2, on the voltammogram you sketched on the
previous page.
Let’s now look at the effect
of changing the concentration of the Cu2+ ions will have on the
voltammogram.
Run the simulation:
·
Display/ Option ®
Overlap (this enables the two voltammograms to be displayed on the same plot)
·
Input/ Chemicals ®
Canal(A) = 2e-3
·
Run/ Simulate
What has happened to id
and E1/2? Find out:
·
Analysis/ Find Halfwave E
Sketch
the voltammograms for the 1 x 10-3 M and 2 x 10-3 M solutions on the same axes.
You may well have expected to
see a change in E1/2 with concentration since this is what is seen
in potentiometry (as described by the Nernst equation). In voltammetry, E1/2 is
unchanged by concentration, and it is id that is concentration
dependent.
Try
another couple of concentrations, tabulating the data (to 3 sig. figs. only)
below:
Now
plot id versus concentration using the data you collected in the
simulations in the space below.
What do you notice?
The linear relationship
between id and concentration can be used as an analytical tool to
determine the concentration of an electroactive species in solution. But how do we know what electroactive
species is/are present? It's the
value of E1/2 that can indicate this. As you know, redox couples have distinct reduction
potentials. The half-wave
potential, E1/2 is very closely related to the Eo values.
Let’s look at the
voltammogram for the reduction of Pb2+ (Eo = - 0.40 V vs.
SCE).
Run the simulation
·
Display/ Option ®
Overlap off
·
Input/ Chemicals ®
Canal(A) = 1e-3
·
Input/ Instrument ®
Eend = -0.6 V
·
Run/ Simulate
·
Display/ Option ®
Overlap on
·
Input/ Chemicals Eo = - 0.40
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Draw
voltammograms for Cu2+ and Pb2+ on the same axes. E1/2 can be used for
identification.
Next we look at the effect of
changing the number of electrons (n) involved in the reduction process. What do you think will happen to the
voltammogram as you increase the number of electrons? Will the current that flows increase, decrease, or stay the
same? Will the diffusion limited
current, id, increase, decrease, or stay the same? Will there be any effect on E1/2?
Do a series of experiments to
investigate the effect of n:
·
Display/ Option ®
Overlap off
·
Input/ Techniques ®
DC voltammetry
·
Input/ Chemicals ®
Eo = 0.1
·
Input/ Instrument ®
Eend = - 0.3 V
·
Input/ Mechanism ®
A + 1e®B
(default setting)
·
Run/ Simulate
·
Display/ Option ®
Overlap on
·
Input/ Mechanism ®
A + 2e®B
·
Run/ Simulate
·
Input/ Mechanism ®
A + 3e®B
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Draw
a series of voltammograms for n = 1, 2 and 3.
The diffusion limited
current, id, increases linearly with n. The shape of the
voltammogram is given by the equation:
E = E1/2 + (0.0592/n)*log [(id - i)/i]
So, as n is increased the
voltammetric wave (i versus E) becomes steeper.

The current is sampled near
the end of the pulse when the Faradaic current (due to the reduction of Cu2+)
is large compared to the charging current (due to the migration of the
electroinactive supporting electrolyte ions in response to the charging of the
electrode) – ie., the signal-to-noise ratio is largest.
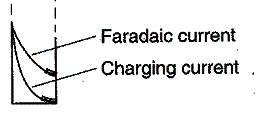
Run the simulation:
·
Display/ Option ®
Overlap off
·
Input/ Chemicals ®
D = 1e-5
·
Run/ Simulate
·
Display/ Option ®
Overlap on
·
Input/ Techniques ®
Normal pulse voltammetry
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Draw
the DC and NP voltammograms on the same scale.
Were you expecting a similar
shape wave? Has E1/2
moved? How do the id's
compare? Because the current is
sampled near the end of the pulse when the Faradaic current (due to the
reduction of Cu2+) is large compared to the charging current (due to
the migration of ions in response to the charging of the electrode), the signal
is increased (as you can see). But
also the noise is decreased and hence the signal-to-noise ratio is improved by
a factor of about 10. The
detection limit of NP voltammetry is 10-6 M, compared with 10-5
M for DC.
Differential
Pulse Voltammetry
In differential pulse (DP)
voltammetry, small pulses are superimposed on a linear voltage ramp.
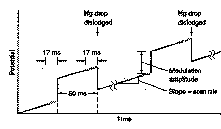
Current is measured before
and after the application of the pulse and the difference in current is
recorded as a function of the applied voltage. The resulting voltammogram is nearly the derivative of a DC
voltammogram. A peak is produced.
Run the simulation:
·
Display/ Option ®
Overlap off
·
Input/ Techniques ®
DC voltammetry
·
Run/ Simulate
·
Display/ Option ®
Overlap on
·
Input/ Techniques ®
Differential pulse voltammetry
·
Run/ Simulate
·
Analysis/ Find Peak
What
peak parameter do you think is most likely to be used in place of E1/2
to identify an electroactive species?
What peak parameter do you expect to be related to the concentration of
the electroactive species?
Indicate these parameters on a sketch of a DP voltammogram (on the next
page).

Run
a series of experiments to test that the peak current, ip, and the peak
area are proportional to concentration, c. Collect data from the simulation
|
[Cu2+]
/ M
|
ip
/ A
|
Peak
Area
|
|
|
|
|
Now
graph ip and
peak area versus c:
The magnitude of the pulse
that is superimposed on the DC ramp in DP voltammetry can be changed. What effect might increasing the size
of this voltage pulse have on driving the reaction and hence on the current? Might any other peak parameters be
affected?
Well, try it and see:
·
Display/ Option ®
Overlap off
·
Input/ Chemicals ®
Canal = 1e-3
·
Input/ Instrument ®
Epulse = - 0.05 (default setting)
·
Run/ Simulate
·
Display/ Option ®
Overlap on
·
Input/ Instrument ®
Epulse = - 0.02
·
Run/ Simulate
·
Input/ Instrument ®
Epulse = - 0.01
·
Run/ Simulate
·
Analysis/ Find Peak
Sketch some voltammograms:
You were probably expecting
to see an increase in the signal, but what else do you notice? Estimate the peak width (in volts) at
half-height. To help you do this,
you could insert gridlines and/or print the voltammograms and measure the peak
widths at half height with a ruler.
What do you notice about the peak
widths? What implications does
this have for resolution of neighbouring peaks?
DP voltammetry allows an even
greater increase in the signal to noise ratio and the detection limit is of the
order of 10-7 M.
Because it is easier to distinguish partially overlapping peaks than
partially overlapping waves, resolution is improved (provided too large a pulse
is not used) from 0.2 V for DC and NP to 0.05 V for DP voltammetry.
Square
Wave Voltammetry
In square wave (SQ)
voltammetry, an alternating (positive, negative, positive, negative….) wave
that is square in shape is superimposed on a voltage staircase.
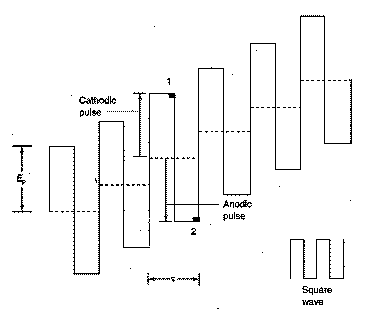
The reverse (anodic) pulse
causes re-oxidation of the product (Cu(s)) of each forward (cathodic)
pulse. The voltammetric signal is
the difference between these two signals and hence will only be recorded when
significant amounts of the oxidised (Cu2+) and reduced forms (Cu(s))
are present.
Run the simulation:
·
Display/ Option ®
Overlap off
·
Input/ Techniques ®
Square Wave voltammetry
·
Run/ Simulate
Sketch the shape of the SQ voltammogram:
Square wave voltammetry is
about 5 times more sensitive than differential pulse. It is also about 100 times faster - an entire square wave
voltammogram can be obtained in less than one second. It can be used for the detection of compounds emerging from
a liquid chromatograph. Compounds
are distinguished by their retention times and E1/2 and are
quantified by peak height or area.
Stripping
Analysis
In stripping analysis, Cu2+
is concentrated onto the electrode (as Cu) by reduction at a potential more
negative (why?) than E1/2.
The copper metal is then stripped off the electrode by reversing the
voltage - ie., sweeping in a
positive direction - driving the oxidation reaction. The current measured during the oxidation is related to the
amount of Cu initially deposited which, under a given set of experimental
conditions, is related to the concentration of Cu2
Run the simulation:
·
Display/ Option ®
Overlap off
·
Input/ Techniques ®
DC voltammetry
·
Run/ Simulate
·
Display/ Option ®
Overlap on
·
Input/ Instrument ®
Pre-concentration on. Note that
the potential will be held at - 0.3 V for 600 s before being reversed and swept
in the positive direction.
·
Run/ Simulate
·
Analysis/ Find Halfwave E
Sketch the shape of the DC stripping
voltammogram.
How does the current measured compare
with that for a normal DC voltammogram?
Think about both the signs and magnitudes of the currents.
Stripping is the most
sensitive of voltammetric techniques (detection limits are ca. 10-10
M) because the analyte is concentrated from dilute solution. The longer the period of concentration,
the more sensitive the analysis.
Confirm this:
·
Input/ Instrument ®
tpre = 1200 s
·
Run/ Simulate
·
Analysis/ Find Halfwave E









